

Ether polyurethane
Polyurethane exhibits a wide range of properties, due to the possibility to modulate the polyols, diisocyanates, catalysts, and additives. The ether-based polyurethane stands out for its resistance to hydrolysis and to microbic attack; it also has optimal mechanical properties at low temperatures and optimal resistance to abrasion. It is resistant to UV rays, if given the appropriate additives, and it is even possible to make it self-extinguishing or conductive. Attention should be given to the chemical resistance of polyurethane, which in general is not high: once the polymer enters into contact with an aggressive substance, the degradative phenomena that are triggered lead to the rupture of the material; in most cases the structural failure of the tube is preceded by swelling. An example of these two phenomena is verified when the polyurethane is in contact with acids and concentrated alkaline solutions, which cause a rapid breakdown of its mechanical properties. Contact with saturated hydrocarbons, diesel gasoline, and kerosene (paraffin), instead, leads to swelling and a reduction in mechanics, but not irreversibly. This phenomenon is reversed once the solution has evaporated and the initial properties are restored.
COMMON NAME
Polyurethane or Thermoplastic Polyurethane
MORPHOLOGY
Semi-crystalline or amorphous polymer
SYNTHESIS
Ether polyurethane is obtained by the polyaddition of ether-based polyols and diols (of varying chain length) with diisocyanates.
STRUCTURE
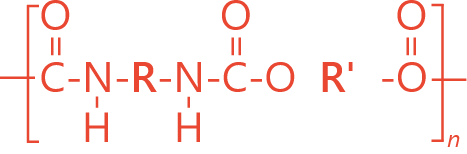
PROPERTIES
-
Dimensional stability
-
Resistance to hydrolysis
-
Resistance to microorganisms
-
Optimal wear and abrasion resistance
-
Good resistance to traction and tearing
-
Optimal capacity for shock absorption
-
Optimal flexibility at low temperatures
-
Excellent impact resistance in the cold
-
Resistance to oils, greases, oxygen, and ozone
-
Lightness
-
Adjustable mechanical properties according to the application
-
Optimal colorability



